"Chinese Solution" Improves The Treatment Effect Of Cerebral Infarction
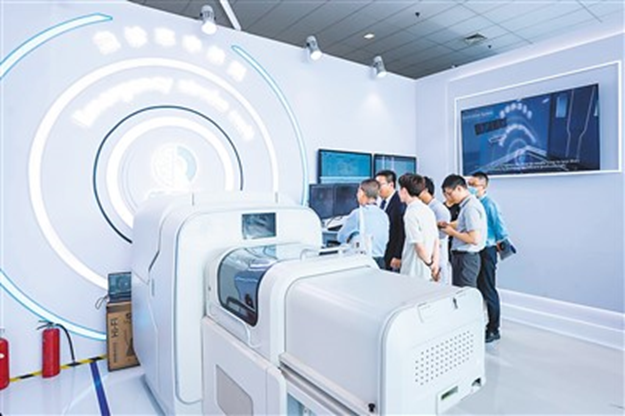
The picture shows the "Emergency Stroke Unit".
Photo courtesy of Beijing Tiantan Hospital
The 10th Annual Academic Meeting of the Chinese Stroke Society and the Tiantan Cerebrovascular Disease Conference (Tiantan Conference) was held in Beijing from June 14 to 16. At the conference, news came that the international authoritative medical journal "New England Journal of Medicine" (NEJM) simultaneously published two heavyweight articles on the treatment of ischemic stroke with domestic thrombolytic drugs reteplase and tenecteplase from Professor Wang Yongjun's team at Beijing Tiantan Hospital affiliated to Capital Medical University on June 15. At the same time, the "Emergency Stroke Unit" with fully independent intellectual property rights, which was created by Beijing Tiantan Hospital, was officially unveiled.
With these new breakthroughs, the treatment of patients with acute ischemic stroke (commonly known as "cerebral infarction") will be more convenient and efficient, and more patients will benefit from it. At the same time, these breakthroughs are expected to change the guidelines for the treatment of acute ischemic stroke and leverage the global thrombolytic drug industry.
This is also the first time that the "New England Journal of Medicine" has simultaneously published two clinical studies of different drugs conducted by the same Chinese team, and it is also the first time that this top journal in the medical field has released research results simultaneously with a Chinese academic conference.
18 mg
"Made in China" has good effect and low cost
In the past 30 years, the "dominant" position of thrombolytic drugs for acute ischemic stroke in the world has always been "occupied" by a drug called alteplase. In 1995, foreign clinical trials confirmed that reperfusion therapy with alteplase intravenous thrombolysis as the core can greatly reduce the disability rate of ischemic stroke and improve patient survival treatment, marking the entry of ischemic stroke into the era of reperfusion therapy. However, this drug monopolized by Western pharmaceutical companies has insufficient supply in many countries around the world due to complex production processes and limited production capacity.
At the same time, as a country with a high incidence of ischemic stroke, China's intravenous thrombolysis rate for the indicated population is only 40%, and there is a significant gap in treatment ratio and quality compared with advanced countries. To reduce the disability and mortality rate due to ischemic stroke, it is necessary to further promote reperfusion therapy with intravenous thrombolysis, and the shortage of thrombolytic drugs has undoubtedly become a huge obstacle to promoting reperfusion therapy in China.
"Since alteplase was launched, the evidence for research on thrombolytic drugs for stroke has basically come from the West, with little data from Asians. There are differences between different races, and Asians directly apply Western data, which leads to uncertainty in the safety and efficacy of drugs." Wang Yongjun, director of Beijing Tiantan Hospital, believes that we need to find a way for the Chinese people, "drugs must be independent, and evidence must be independent."
Reteplase is a genetically engineered modified drug that was approved for marketing abroad in 1996 for the treatment of acute myocardial infarction. From the perspective of drug principles, this drug should have a good effect on acute ischemic stroke, but for many years, due to the "halo" of alteplase, few people have linked reteplase with stroke.
In 2017, Wang Yongjun's team hit it off with a Beijing state-owned enterprise and decided to wake up this drug that had been "sleeping" for more than 20 years.
Li Shuya, director of the Clinical Trial Center of Beijing Tiantan Hospital, is the head of the research project called "RAISE". "This is the world's first and largest Phase III clinical trial comparing the effects of reteplase and alteplase in acute ischemic cerebrovascular events. It was jointly completed by 62 hospitals across the country, with a total of 1,412 patients enrolled." Li Shuya introduced that the test results showed that for patients with acute ischemic stroke who were suitable for intravenous thrombolysis treatment within 4.5 hours of onset, the proportion of patients with good functional prognosis in the reteplase treatment group was better than that in the alteplase group at 90 days, and there was no significant difference in the proportion of patients with symptomatic intracranial hemorrhage and death compared with the alteplase treatment group. "In layman's terms, reteplase is better than alteplase."
Compared with alteplase, reteplase does not require the estimation of the patient's weight or intravenous drip. It only requires two intravenous injections of 18 mg of the drug half an hour apart. More importantly, in order to better adapt to stroke treatment, the research team optimized the production process of this drug, which greatly reduced the cost and greatly reduced the economic burden on patients.
"Next, we will conduct an international multicenter trial of reteplase to allow this "Made in Beijing" drug to go overseas as soon as possible." Wang Yongjun said.
24 hours
Broaden the time window for intravenous thrombolysis
In fact, Wang Yongjun's team found more than just reteplase. A year ago, the Lancet published a study called "TRACE Ⅱ". This study shows that a new generation of specific thrombolytic drug tenecteplase (TNK) independently developed by China and modified by genetic engineering is not inferior to alteplase under the premise of comparable safety.
"This is just the starting point." Wang Yongjun said that there are many and complex factors affecting the proportion of reperfusion therapy in China, and finding drugs cannot solve all problems. "For example, many patients do not understand ischemic stroke, do not go to the hospital for treatment in time after the onset, or cannot judge the time of onset; some primary medical institutions do not have the conditions and capabilities for intravenous thrombolysis, and patients need to be transferred to hospitals that can thrombolyze, wasting time on the road..."
Data show that about 67% to 75% of acute ischemic stroke patients arrive at the hospital more than 4.5 hours or the onset time is unknown. Time is the key to saving patients' lives and avoiding disability as much as possible. Even after the continuous efforts of scientific researchers, the time window for intravenous thrombolysis of acute ischemic stroke is only 4.5 hours.
Can a breakthrough be made? Wang Yongjun and his team members set their sights on this "4.5 hours".
"We selected patients with acute ischemic stroke who had large blood vessel occlusion but imaging showed the presence of a 'penumbra' in the brain 4.5 to 24 hours after onset, and used tenecteplase for treatment." Project executive leader, Xiong Yunyun, deputy director of the Department of Cardiovascular Neurology at the Neurology Center of Beijing Tiantan Hospital, said that the "penumbra" means that this part of the brain tissue is between normal and infarction, and the function may still be restored after normal blood flow is restored, which can save patients from disability or reduce the degree of disability.
This study, called "TRACE Ⅲ", introduced artificial intelligence technology. In order to make imaging judgments more timely and accurate, the team independently developed the iStroke acute stroke intelligent imaging decision-making platform. As an "assistant", this system can quickly analyze and evaluate head images to find the key "penumbra".
This trial included 516 patients from 58 research centers across the country, and the final conclusion was exciting: "For patients with acute ischemic stroke who have anterior circulation large artery occlusion and imaging penumbra within 4.5-24 hours after onset, the use of intravenous thrombolysis with tenecteplase can reduce the disability rate, and does not increase the mortality rate and the number of patients with symptomatic intracranial hemorrhage."
"This is the first time in the world that it has been confirmed that intravenous thrombolysis is safe and effective when the time window is extended to 24 hours." Xiong Yunyun introduced that among stroke patients worldwide, a large proportion of patients have large artery occlusion within 24 hours of onset and have salvageable brain tissue, but cannot receive endovascular treatment for various reasons. The "TRACE Ⅲ" trial provides a new late time window intravenous thrombolysis treatment for such patients. At the same time, the convenient administration of tenecteplase intravenous injection is expected to reduce the risk of stroke progression during inter-hospital transfer.
32 square meters
Maximize time to protect the brain
"In the traditional medical model, stroke patients need to undergo clinical evaluation, CT, MRI and other imaging evaluations after arriving at the emergency room. It often takes about 1 hour from entering the hospital to receiving thrombolytic therapy. For patients, the simpler the links and the smoother the process, the shorter the waiting time, and the more brain function can be protected." Wang Yongjun said that it was for this goal that this 32-square-meter "Emergency Stroke Unit" was established.
This "Emergency Stroke Unit" highly concentrates the key links of traditional acute stroke clinical evaluation, imaging evaluation and treatment into one space, and adds innovative solutions, which can be called "hidden dragons and crouching tigers".
Quick physical examination. It is equipped with intelligent wearable devices that can collect key information such as blood pressure and blood sugar of patients in real time and monitor patients' vital signs.
"Elf". This completely independently developed low-field strength MRI scanner has a field strength of only 0.23 Tesla (conventional MRI is 1.5 or 3.0 Tesla), uses 220V household power, and can be easily moved. More importantly, patients do not need to remove any metal objects they carry or implant, and can be examined directly, which greatly saves time. Through the independently developed artificial intelligence image recognition system, cerebral hemorrhage and cerebral ischemia can be quickly identified within 1 and a half minutes, ensuring that patients receive effective treatment in the shortest time.
Information technology assists diagnosis. Here, full structured data can be quickly entered. Based on the patient's physical signs and imaging manifestations, AI can assist medical staff in making decisions.
Innovative drug treatment. The application of new generation thrombolytic drugs such as tenecteplase and reteplase, intravenous injection replaces the previous one-hour intravenous drip, and the drug works faster.
Through the "Emergency Stroke Unit", the time from the patient's arrival at the hospital to receiving reperfusion treatment can be compressed to within 20 minutes, and there is no need to run around the clinic, imaging examination, laboratory, treatment room, etc., to maximize the time to protect the patient's brain function.
At present, the "Emergency Stroke Unit" has been put into use in nearly 20 hospitals in China.
"The key word of these projects is 'self-reliance'. my country is a major country with ischemic stroke. We must develop drugs, devices and equipment suitable for Chinese people, rewrite more guidelines, make the treatment of ischemic stroke faster and more convenient, and allow more ischemic stroke patients to receive timely and effective treatment." Wang Yongjun said.